Autoři: Radek Ptáček, Michal Goetz
Garant: Michal Goetz
Oponent: Petra Uhlíková
Vysvětlivky:
ovál - nadpis
kosočtverec - výběr z více variant
přerušovaná čára - diagram pokračuje na další straně
Závěrečný komentář
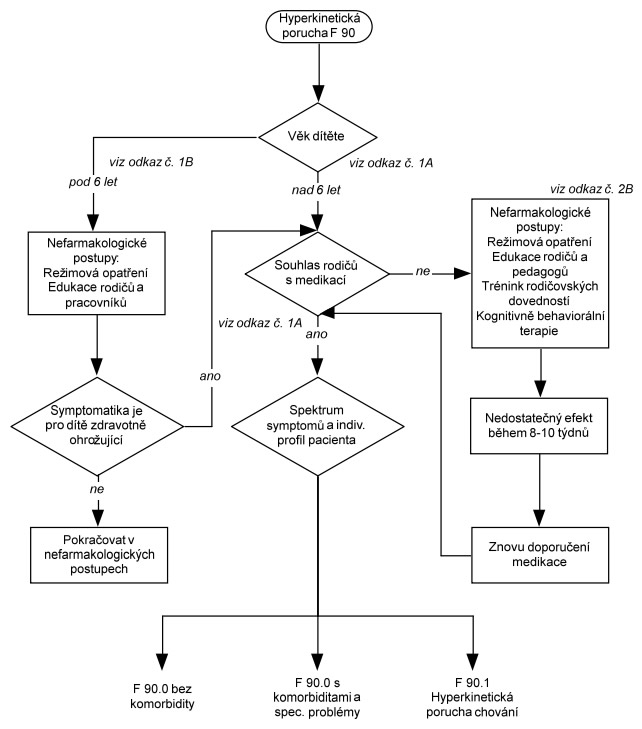
Vodítka léčby HKP vycházejí z mezinárodních vodítek a jsou rozdělena do dvou oddělených schémat. Schéma 1 se týká Poruchy aktivity a pozornosti, a zahrnuje dva odlišné postupy: první u nekomplikované formy poruchy, druhý u poruchy s komorbiditami a specifickými problémy. Schéma 2 pojednává léčbu Hyperkinetické poruchy chování a představuje jediný postup.
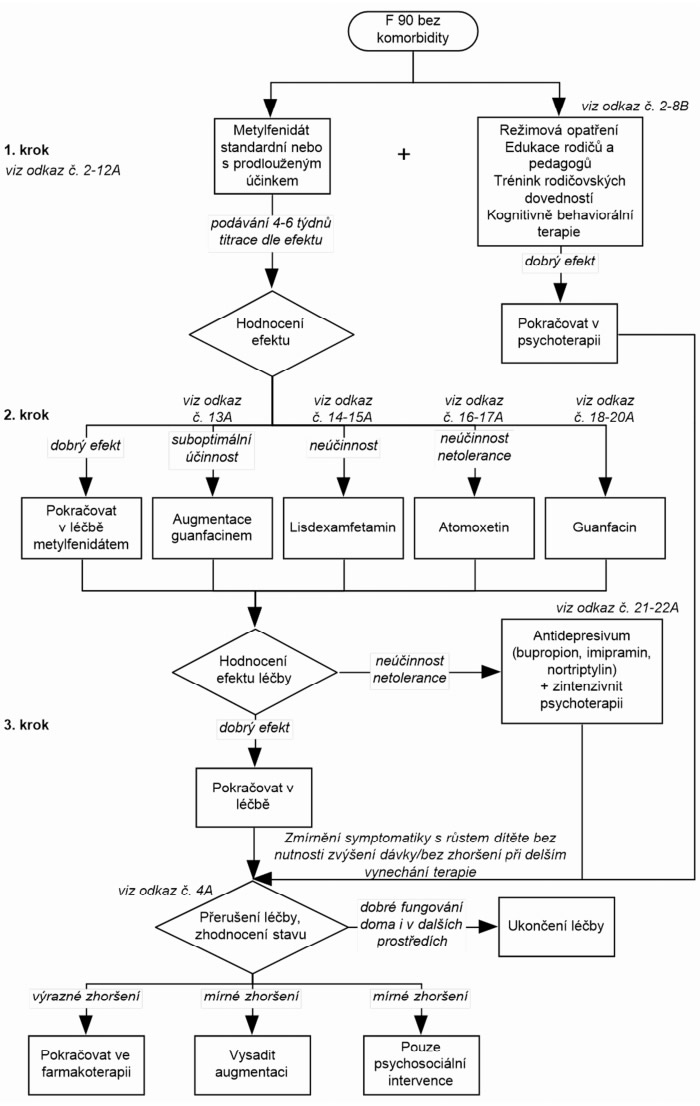
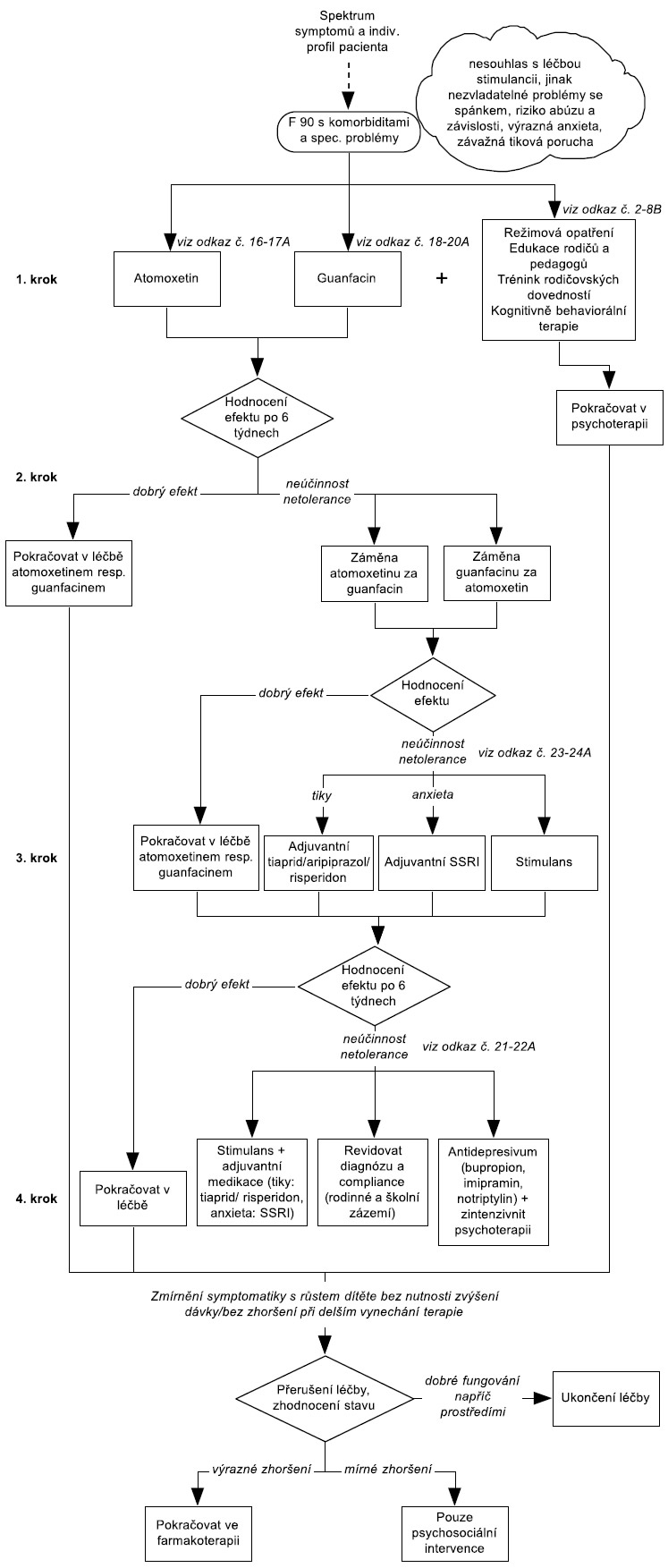
Ke schématu 1: Při formulaci léčebných postupů u Poruchy aktivity a pozornosti je možné vyjít z doporučených postupů léčby pro děti s AD/HD bez komorbidity podle DSM-5. Oba klasifikační systémy považují pro diagnózu za určující shodné symptomy, i když podle DSM-5 tyto symptomy mohou - ale také nemusejí - být přítomny vždy současně. Je nutná pouze modifikace vzhledem k rozdílnému sortimentu registrovaných preparátů v příslušných zemích. V naší republice je možné volit formu metylfenidátu s okamžitým uvolňováním, nebo s prodlouženým uvolňováním a nově je registrován též lisdexamfetamin jež má prodloužený účinek. Individuální volba musí zohlednit compliance pacienta, aby bylo zajištěno spolehlivé zajištění lékem během dne. Jestliže rodiče léčbu stimulancii odmítají nebo jsou kontraindikována nebo jsou přítomny některé komorbidní poruchy (tiky, výrazná anxieta, riziko vzniku závislostí), měl by být u F90.0 použit atomoxetin nebo guanfacin.
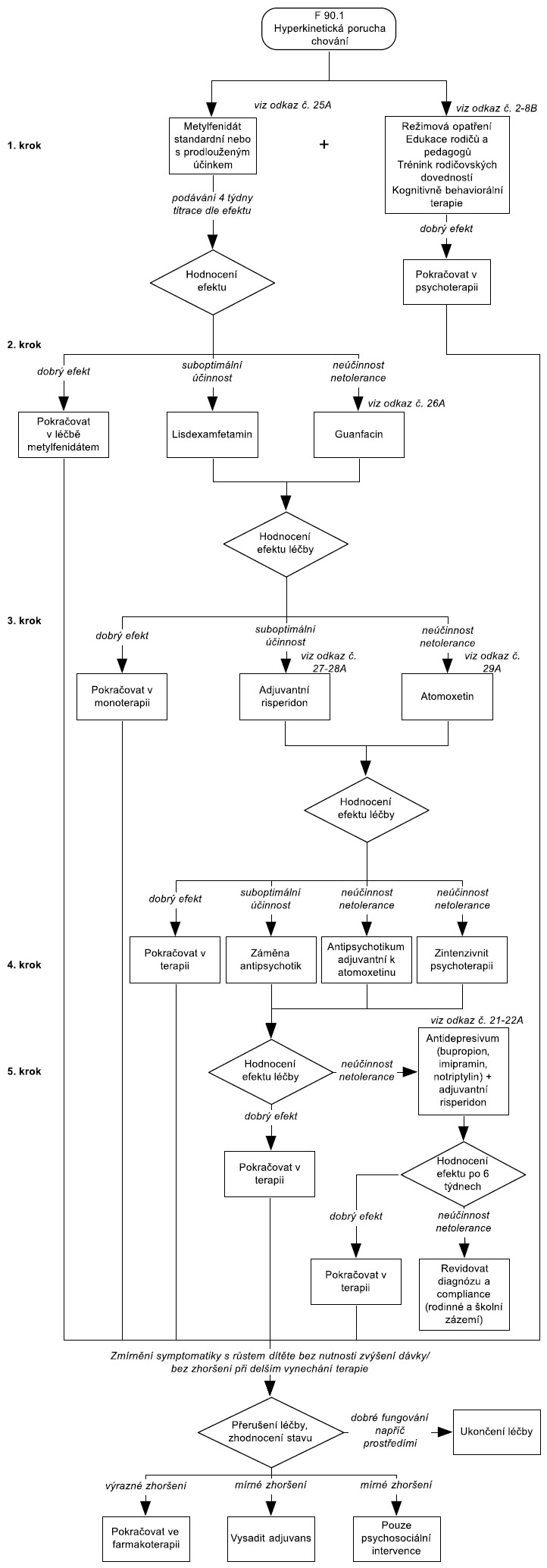
Ke schématu 2: Pro léčbu hyperkinetické poruchy chování (podle MKN-10) lze podle názoru expertů pracovní skupiny Global ADHD Working Group (Remschmidt, 2005) aplikovat postup, který je ve standardech pro léčbu ADHD uváděn pro případy komorbidního výskytu ADHD s poruchami chování. Pokud však u hyperkinetické poruchy chování (podle MKN-10) nejsou poruchy chování příliš závažné (na příklad není přítomna závažná agresivita a disociální jednání) a behaviorální problémy splňují spíše kritéria pro poruchu opozičního vzdoru, lze také aplikovat doporučený postup léčby platný pro poruchu pozornosti s hyperaktivitou ( resp. pro AD/HD bez komorbidity).
V posledních letech byla publikována řada meta analytických studií, které se zabývají účinností nefarmakologických postupů (např.: Arns a kol., 2009; Sonuga-Barke a kol., 2013; Catala-Lopez a kol., 2017). Tyto práce poukazují na metodologické nedostatky klinických studií u non-farmakologických postupů a malý počet studí. Není tak v řadě případů doposud popsaná přesná indikace, standardizované postupy ani dostatečně velikost efektu ve srovnání s jinými metodami.
Nicméně, současná meta-analýza uzavírá, že behaviorální terapie zejména je-li poskytována rodiči s aktivním zapojením dítěte a učitele je jediným nefarmakologickým postupem, který prokázal statisticky signifikantní efekt na zmírnění symptomů poruchy (Catala-Lopez a kol., 2017)
Kognitivní trénink, neurofeedback, dietní opatření, užívání nenasycených mastných kyselin, aminokyselin, minerálů, bylinná terapie a fyzická aktivita nemohou být označeny jako prokazatelně účinná a specifická léčba poruchy pozornosti s hyperaktivitou (Sonuga-Barke et al. 2013,Lofthouse et al. 2012, Catala-Lopez a kol., 2017 ).
Reference
1. Allen, A.J., Kurlan, R.M., Gilbert, D.L., Coffey, B.J., Linder, S.L., et al.: Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorder. Neurology, 65, 2005, pp.1941 - 1949.
2. Aman, M.G., DeSmedt,G., Derivan, A., Lyons, B., Findling, R., et al.: Risperidone treatment of children with disruptive behavior symptoms and subaverage IQ: a double - blind placebo - controlled study. Am. J. Psychiatry, 159, 2002, s.1337 - 1346.
3. Aman, M.G., Binder, C., Turgay, A.: Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavior disorder and subaverage IQ. J. Child Adolesc Psychopharmacol., 14, 2004, s.243 - 254.
4. American Academy of Child and Adolescent Psychiatry (AACAP): Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry, 46, 2007, s.894 - 921.
5. Barrickman, L.L., Perry, P.J., Allen, A.J., Kuperman, S., Arndt, S.V., et al.: Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry, 34, 1995, s.649 - 657
6. Biederman J, Melmed RD, Patel A, McBurnett K, Donahue J, Lyne A. Long-term, open-label extension study of guanfacine extended release in children and adolescents with ADHD. CNS Spectr. 2008;13:1047–55.
7. Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–84.
8. Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA): Canadian ADHD Practice guidelines, Third Edition. Toronto, CADDRA 2011.
9. Catalá-López F, Hutton B, Núñez-Beltrán A, Page MJ, Ridao M, Macías Saint-Gerons D, Catalá MA, Tabarés-Seisdedos R, Moher D. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. PLoS One. 2017 Jul 12;12(7):e0180355.
10. Coghill DR, Caballero B, Sorooshian S, Civil R. A systematic review of the safety of lisdexamfetamine dimesylate. CNS Drugs. 2014 Jun;28(6):497-511. doi: 10.1007/s40263-014-0166-2.
11. Cohen SC, Mulqueen JM, Ferracioli-Oda E, Stuckelman ZD, Coughlin CG, Leckman JF et al. Meta-Analysis: risk of tics associated with psychostimulant use in randomized, placebo-controlled trials. J Am Acad Child Adolesc Psychiatry 2015 Sep;54(9):728–736.
12. Conners, C.K., Casat, C.D., Gualtieri, C.T., Weller, E., Reader, M., et al.: Bupropion hydrochloride in attention deficit disorder with hyperactivity. J. Am. Acad. Child Adolesc. Psychiatry, 35, 1996, s.1314 - 1321.
13. Connor DF, Findling RL, Kollins SH, Sallee F, Lopez FA, Lyne A, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6–12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a random- ized, double-blind, placebo-controlled trial. CNS Drugs. 2010;24:755–68.
14. Cortese, S., Faraone, S.V., Konofal, E., & Lecendreux, M. (2009). Sleep in children with attention-deficit/hyperactivity disorder: Meta-analysis of subjective and objective studies. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 894–908.
15. Cortese S, Holtmann M, Banaschewski T, et al. Practitioner Review: Current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. Journal of Child Psychology and Psychiatry. 2013;54(3):227-246.
16. Geller, D., Donelly, C., Lopez, F., Rubin, R., Newcorn, J., et al.: Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry, 46, 2007, s.1119 - 1127.
17. Gillies D, Sinn JK, Lad S, Leach MJ, Ross MJ: Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database SystRev 2012; 7:CD007986
18. Graham, J., & Coghill, D. (2008). Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: Epidemi- ology, prevention and management. CNS Drugs, 22, 213–237.
19. Gualtieri, C.T., Evans, R.W.: Motor performance in hyperactive children treated with imipramine. Percept. Mot. Skills, 66, 1988, s.763 - 769.
20. Hasnain M, Vieweg WV. Weight considerations in psychotropic drug prescribing and switching. Postgrad Med. 2013;125:117–29.
21. Hodgkins P, Shaw M, Coghill D, Hechtman L. Amfetamine and methylphenidate medications for attentionde cit/hyperactivity disorder: complementary treatment options. Eur Child Adolesc Psychiatry. 2012;21:477–92.
22. Huss M, Chen W, Ludolph AG. Guanfacine Extended Release: A New Pharmacological Treatment Option in Europe. Clinical Drug Investigation. 2015;36(1):1-25. doi:10.1007/s40261-015-0336-0.
23. Konofal, E., Lecendreux, M., & Cortese, S. (2010). Sleep and ADHD. Sleep Medicine, 11, 652–658.
24. Kutcher, S., Aman, M., Brooks, S.J., Buitelaar, J., van Daalen, E., et al.: International consensus statement on attention-deficit/hyperactivity disorder (ADHD) and disruptive behaviour disorders (DBDs): clinical implications and treatment practice suggestions. Eur. Neuropsychopharmacol., 14, 2004, s.11 - 28.
25. Loy JH, Merry SN, Hetrick SE, Stasiak K. Atypical antipsychotics for disruptive behaviour disorders in children and youths. Cochrane Database Syst Rev. 2017 Aug 9;8:CD008559.
26. Maneeton B, Maneeton N, Likhitsathian S, Suttajit S, Narkpongphun A, Srisurapanont M, Woottiluk P. Comparative efficacy, acceptability, and tolerability of lisdexamfetamine in child and adolescent ADHD: a meta-analysis of randomized, controlled trials. Drug Des Devel Ther. 2015 Apr 1;9:1927-36
27. , A.T., Holdridge, K.C., Johannes, C.B., Hornbuckle, K., & Walker, A.M. (2008). The effect of pharmacotherapy for attention deficit hyperactivity disorder on risk of seizures in pediatric patients as assessed in an insurance claims database. Current Drug Safety, 3, 123–131.
28. Michelson, D., Faries, D., Wernicke, J., Kelsey, D., Kendrick, K., et al.: Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics, 108, 2001, s.83 - 91.
29. MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry, 56, 1999, s.1073-1086.
30. Murphy AL, Gardner DM, Kisely S, Cooke CA, Kutcher SP, Hughes J. System struggles and substitutes: A qualitative study of general practitioner and psychiatrist experiences of prescribing antipsychotics to children and adolescents. Clin Child Psychol Psychiatry. 2016 Oct;21(4):634-648.
31. National Institute for Health and Clinical Excellence (NICE): Attention deficit hyperactivity disorder. NICE clinical guideline 72. London, NICE 2008.
32. Newcorn JH, Stein MA, Childress AC, Youcha S, White C, Enright G, Rubin J. Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: morning or evening administration. J Am Acad Child Adolesc Psychiatry. 2013 Sep;52(9):921-30.
33. Newcorn JH, Harpin V, Huss M, Lyne A, Sikirica V, Johnson M, Ramos-Quiroga JA, van Stralen J, Dutray B, Sreckovic S, Bloomfield R, Robertson B. Extended-release guanfacine hydrochloride in 6-17-year olds with ADHD: a randomised-withdrawal maintenance of efficacy study. J Child Psychol Psychiatry. 2016 Jun;57(6):717-28.
34. Olfson M, King M, Schoenbaum M (2015) Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry 72:867–874
35. Prasad, S., Steer, C.: Switching from neurostimulant therapy to atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder. Pediatr. Drugs, 10, 2008, s.39 - 47.
36. Pringsheim T, Doja A, Gorman D, McKinlay D, Day L, Billinghurst L, et al. Canadian guidelines for the evidence-based treatment of tic disorders: pharmacotherapy. Can J Psychiatry. 2012;57:133–43.
37. Pringsheim T, Hirsch L, Gardner D, Gorman DA. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 1: psychostimulants, alpha-2 agonists, and atomoxetine. Can J Psychiatry. 2015 Feb;60(2):42-51.
38. Reed VA, Buitelaar JK, Anand E, Day KA, Treuer T, Upadhyaya HP, Coghill DR, Kryzhanovskaya LA, Savill NC. The Safety of Atomoxetine for the Treatment of Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Comprehensive Review of Over a Decade of Research. CNS Drugs. 2016 Jul;30(7):603-28.
39. Remschmidt, H. by the Global ADHD Working Group: Global consensus on ADHD/HKD. Eur. Child Adolesc. Psychiatry, 14, 2005, č.3, s.127 - 137.
40. Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067–74.
41. Seixas, M., Weiss, M., Muller, U.: Systematic review of national and international guidelines on attention-deficit hyperactivity disorder. J. Psychopharmacol., 26, 2012, s. 753 - 765.
42. Schachar, R., Tannock, R., Cunningham, C. , Corkum, P.: Behavioral, situational, and temporal effects of treatment of ADHD with methylphenidate. J. Am. Acad. Child Adolesc. Psychiatry, 36, 1997, s.754 - 763.
43. Schröder C, Dörks M, Kollhorst B, Blenk T, Dittmann RW, Garbe E, Riedel O. Outpatient antipsychotic drug use in children and adolescents in Germany between 2004 and 2011. Eur Child Adolesc Psychiatry. 2017 Apr;26(4):413-420. doi: 10.1007/s00787-016-0905-7.
44. Sonuga-Barke E, Dittmann R, Simonoff E et al. European ADHD Guideline Group. Nonpharmacological Interventions for ADHD: Systematic Review and Meta-Analyses of Randomized Controlled Trials of Dietary and Psychological TreatmentsAm J Psychiatry 2013; 170:275–289
45. Spencer, T., Heiligenstein, J.H., Biederman, J., Faries, D.E., Kratochvil, C.J., et al.: Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J. Clin. Psychiatry, 63, 2002, s.1140 - 1147.
46. Spencer TJ, Greenbaum M, Ginsberg LD, Murphy WR. Safety and effectiveness of coadministration of guanfacine extended release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:501–10.
47. Storebø OJ, Krogh HB, Ramstad E, Moreira-Maia CR, Holmskov M, Skoog M, Nilausen TD, Magnusson FL, Zwi M, Gillies D, Rosendal S, Groth C, Rasmussen KB, Gauci D, Kirubakaran R, Forsbøl B, Simonsen E, Gluud C. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ. 2015 Nov 25;351:h5203.
48. Swanson, J.M., Wigal, S.B., Wigal, T., Sonuga- Barke, E., Greenhill, L.L., et al.: A comparison of once-daily extended -release methylfenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (The Comacs study). Pediatrics, 113, 2004, s.206 - 216.
49. SPC Concerta,datum revize textu 6.9.2011
50. SPC Strattera.datum revize textu 3.5.2013,
51. SPC Elvanse, datum revize textu 27.10.2016
52. SPC Intuniv, Datum první registrace: 17. září 2015
53. Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH. Systematic review: pharmacological treatment of tic disorders— efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev. 2013;37:1162–71.
54. Weiss, M.D., Wasdell, M.B., Bomben, M.M., Rea, K.J., & Freeman, R.D. (2006). Sleep hygiene and melatonin treat- ment for children and adolescents with ADHD and initial insomnia. Journal of the American Academy of Child and Adolescent Psychiatry, 45, 512–519.
55. Wilens TE, Robertson B, Sikirica V, Harper L, Young JL, Bloomfield R, Lyne A, Rynkowski G, Cutler AJ. A Randomized, Placebo-Controlled Trial of Guanfacine Extended Release in Adolescents With Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2015 Nov;54(11):916-25
56. Wilens TE, Bukstein O, Brams M, Cutler AJ, Childress A, Rugino T, Lyne A, Grannis K, Youcha S. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012 Jan;51(1):74-85.e2.
57. Wilens, T., Biederman, J., Geist, D.E., Steingard, R., Spencer, T.: Nortriptyline in the treatment of attention deficit hyperactivity disorder: a chart review of 558 cases. J. Am. Acad. Child Adolesc. Psychiatry, 32, 1993, s.343 - 349.
58. Zito JM, Burcu M. Stimulants and Pediatric Cardiovascular Risk. J Child Adolesc Psychopharmacol. 2017 Aug;27(6):538-545. doi: 10.1089/cap.2015.0239. Epub 2016
59. American Academy of Pediatrics. (2010). Appendix S2: Evidence-Based Child and Adolescent Psychosocial Interventions. Pediatrics, 125(Supplement 3), S128-S128.
Arns, M., de Ridder, S., Strehl, U., Breteler, M., & Coenen, A. (2009). Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clinical EEG and neuroscience, 40(3), 180-189.
60. Catala-Lopez, F., Hutton, B., Núñez-Beltrán, A., Page, M. J., Ridao, M., Saint-Gerons, D. M. & Moher, D. (2017). The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. PLoS one, 12(7), e0180355.
61. Evans, S. W., Owens, J. S., & Bunford, N. (2014). Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder. Journal of Clinical Child & Adolescent Psychology, 43(4), 527-551.
62. Satapathy, S., Choudhary, V., Sharma, R., & Sagar, R. (2016). Nonpharmacological interventions for children with attention deficit hyperactivity disorder in India: A comprehensive and comparative research update. Indian journal of psychological medicine, 38(5), 376.
63. Sonuga-Barke, E. J., Brandeis, D., Cortese, S., Daley, D., Ferrin, M., Holtmann, M., ... & Dittmann, R. W. (2013). Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. American Journal of Psychiatry, 170(3), 275-289.
64. Young, S., & Myanthi Amarasinghe, J. (2010). Practitioner Review: Non‐pharmacological treatments for ADHD: A lifespan approach. Journal of Child Psychology and Psychiatry, 51(2), 116-133
Datum zveřejnění: 10.6.2018
